Plant Operation
Home / Plant Operations
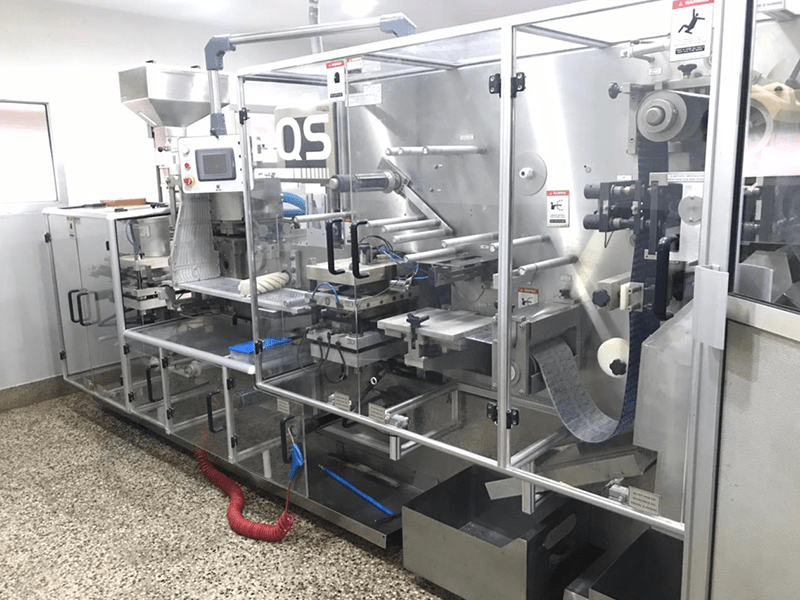
Manufacturing Facility
Efroze uses the state of the art Facilities, standards of quality and environmental and safety practices, interminable operating procedures with highly technical proficient team to produces quality products. These includes solid and liquid dosage forms (Tablet, capsule, liquid syrups/suspensions, dry powder).
Efroze at its high-tech facility (Hormone Section) produces specialty hormone products.
In the plant Operations, we have a highly competent and trained staff of pharmacists and chemists, as well as highly talented engineers and technicians to support them in the production in a continuous improvement environment.
Quality Control
Efroze Chemical industries has a Dedicated, highly developed Quality control laboratory with particularly advanced Instruments for the testing & analysis, which ensures that there is no compromise over quality of products.
Quality Assurance
Our Quality Assurance department ensures the highest level of quality, safety, and efficacy of products with highly qualified & skilled professionals .We have a comprehensive Quality Management System to ensure quality excellence in the organization..
Quality Policy
We are committed to achieving Quality Management System (QMS) requirements, which are commensurate with internationally recognized management system standards, in order to achieve professional excellence in the pharmaceutical sector.
By evaluating organizational objectives, satisfying consumers, and complying with legal and other applicable standards, we will maintain the efficacy and continuous improvement of our QMS.
We encourage our employees to improve their skills and quality awareness.
Product Development
We are dedicated to the development of new products in order to provide the most cutting-edge compounds for well-being.
Our Product development Department was built with “highly qualified and skilled people” who are professionals in designing and developing unique formulations.
- Working on new product development is our project.
- Analytical techniques are being developed.
- Validation Methods are being developed.
- Following the ICH Guidelines for Stability Studies.
Product development fully equipped with high-tech machinery that can handle the following forms effectively:
Tablet (Controlled Release, Immediate Release and Enteric Coated).
Capsule
Suspension/Syrup
Dry powder sachet
Product development team aims at continuous advancement in new product development which ensures the accessibility of every formulation.

